Business
Essential Insights into the Rising Threat of Bird Flu

In early 2024, a troubling development occurred as highly pathogenic avian influenza (H5N1) unexpectedly infected dairy cows in the Texas Panhandle. Traditionally associated with birds, this virus now poses a potential threat to mammals, which could enable further cross-species transmission. “Holy cow,” remarks Thomas Friedrich, a virologist from the University of Wisconsin–Madison. “This is how pandemics start.”
H5N1 has reached panzootic status, impacting species across every continent except Australia and infecting animals ranging from cats to dolphins. While the United States is the only country reporting infections in cows, dairies in at least 17 states have confirmed cases. “This is the largest animal disease outbreak we’ve ever had,” states Maurice Pitesky, a veterinary researcher at the University of California, Davis.
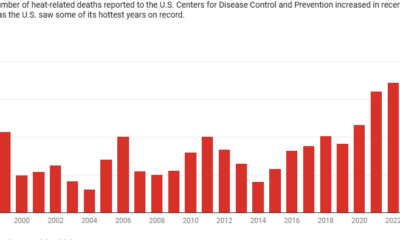
H5N1, which has caused upheaval in the poultry industry, has begun affecting dairy operations, influencing the economic landscape due to fluctuating egg prices and threatening food security. Notably, the virus has infected over 960 people worldwide since 2003, resulting in a mortality rate of nearly 50%. In early 2025, it claimed its first life in the U.S., with several farm workers already reported infected.
Currently, the Centers for Disease Control and Prevention and the World Health Organization regard the public health risk as low, given that H5N1 has not yet developed the ability for efficient human-to-human transmission. However, experts like Michelle Wille emphasize the unpredictability of the virus, stating, “Every time we think we know what’s going to happen, it does something totally unexpected.”
The biological components of H5N1 are not fundamentally different from other influenza A strains. It consists of eight segments of genetic material encoding 11 proteins, with hemagglutinin (H) and neuraminidase (N) being pivotal for its infectious capabilities. Variability in these proteins creates different strains, allowing the potential for cross-species transmission.
Historically, scientists predicted that avian influenza viruses would generally result in isolated outbreaks that could die off rather than spill over into humans. However, following the first recorded human cases of H5N1 in Hong Kong in 1997, the situation proved more complex as the virus continued to elude expectations, infecting numerous species and subsequently adapting.
After confirming the existence of H5N1 in dairy cows, a variety of biosecurity measures are being suggested for farmers, including limiting human interaction and tightening sanitation practices. The USDA now mandates testing for H5N1 across all dairy operations in the contiguous states. Recently, additional outbreaks were reported in Nevada and Arizona, involving a different strain than previously observed.
Long-term effects on cattle remain uncertain, prompting organizations like the National Milk Producers Federation to express the need for effective vaccines. Zoetis is actively researching vaccines specific to H5N1 for cows, though their deployment remains unclear.
Despite potential surveillance and prevention measures, experts like Gregory Gray caution that these viruses may become endemic. However, as of early 2025, there have been no confirmed human-to-human transmissions in the U.S. Gray elucidates, “It’s going to take continual spillover events for it to get a foothold.” To adapt for efficient human transmission, significant genetic changes would be required.
The developing situations in agriculture provide a double-edged sword; while vaccines and protective measures are being developed, the overall risk from H5N1 to humans requires constant monitoring. Factors affecting transmissibility, including historical immunity levels in the human population, may help mitigate severe impacts but can’t eliminate risk entirely.
Impacts on wildlife are developing rapidly, with thousands of birds and mammals reported affected. H5N1’s reach has extended down the Pacific coast of South America, leading to alarming mortality rates among various species. Yet, as Wille notes, “I think we’re on the precipice of something. What that something is, I’m not sure.” The next steps in managing H5N1 will require global cooperation and readiness for unexpected developments in this ongoing animal health crisis.